Astrochemistry: Alchemy in Space
Astronomy Chemistry
This is the Butterfly Nebula
(NGC 6302) captured by the Hubble Space Telescope.
Why do you think it appears red? Well, it’s red due to the spectral emission of Hydrogen atoms that have been energised by the heat from the star. The hints of other colours are due to the presence of other elements inside the cloud.
So how do we know all this? Well, credits to Astrochemistry.
Astrochemistry is a branch of astronomy that deals with the study of the chemical composition of the universe. Whether to find the composition of an asteroid or that of a galaxy, astrochemistry can do it all. The history of astrochemistry is shared by the field of astronomy and chemistry. With the advancement in observational and experimental spectroscopy, these two fields found a way to co-exist giving us this ever-growing field of astrochemistry.
What is done in this field?
Astrochemists are trying to understand the composition (chemical) of the heavenly bodies through data collected by various satellites, telescopes, space vehicles, etc. They learn about the compositions of various stars, comets, planets, interstellar media, and many more.
-
They research about:
- How atoms and molecules interact outside of the earth's atmosphere.
- Understand the geological composition of other planets.
- Examine other planets for habitability and life.
- Composition of the stars and other bodies.
Current and upcoming observatories are designed to detect the chemical elements making up planetary atmospheres, looking for molecules like water and carbon compounds. Simple molecular species, carbon chains, complex organic molecules (COMs), fullerenes, and polycyclic aromatic hydrocarbons (PAHs) are omnipresent in the space environment. Molecules have been detected at every stage of stellar evolution and in regions and situations that might seem inhospitable to the formation and survival of chemical bonds.
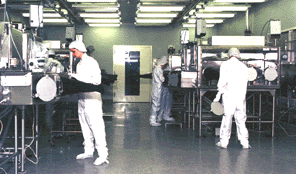
The Lunar samples collected during the 6 Apollo missions
from 1969-1972 revealed the early history of the moon, the earth and the inner
solar system.
NASA JSC File Photograph S85-36332. Staff scientists at work in the Lunar Sample
Laboratory Facility at NASA JSC. "Pristine" lunar samples (those continuously in
NASA custody since return from the Moon) are stored and handled in stainless steel
glove cabinets that are purged by high-purity nitrogen gas to minimise degradation
of the samples. Pristine samples are always separated from human hands by three layers
of gloves.
-
Some results from the data include:
- Lunar soil being very dry lacks the minerals with water as part of their structure (mineral hydration) such as clay, mica, and amphiboles
- The iron in the lunar soil is found in elemental form, which is rare to find on earth.
Astrochemistry being a research-oriented field, a PhD with concentrations in both chemistry and astronomy is required.
-
Some basic skill set that a candidate must possess in this field:
- A solid background in chemistry (or a related scientific field) and astronomical data collection and analysis.
- Understanding of various astronomical concepts.
- Understanding of theoretical principles, including kinetics, thermodynamics, and quantum chemistry.
- Familiarity with computer modelling and statistical analysis methods.
Some interesting research and Discoveries
One of the important discoveries made in 2018, is the detection of Benzonitrile in the Taurus molecular cloud (TMC-1), which is 430 light-years away, using the NSF’s Green Bank Telescope, a radio telescope by a research team under chemist Dr Brett McGuire of the National Radio Astronomy Observatory.
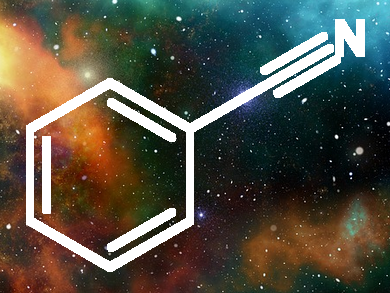
Benzonitrile’s lopsided chemical arrangement allowed the chemists to identify
nine distinct spikes in the radio spectrum that correspond to the molecule. They also could
observe the additional effects of nitrogen nuclei on the radio signature.
This discovery is a vital clue in a 30-year-old mystery: identifying the source of a faint
infrared glow that permeates the Milky Way and other galaxies.
Another interesting discovery that answers one important question in astrochemistry: how to
measure magnetic fields in space using methanol? A team of scientists, led by Boy Lankhaar at
Chalmers University of Technology, in 2018 has found a way to do it.
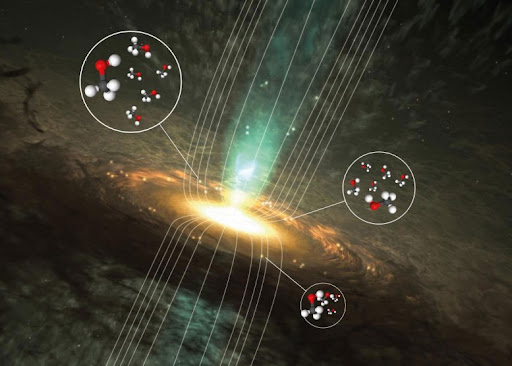
This illustration shows the surroundings of a forming massive star and the bright regions where radio signals from methanol can be found. The use of methanol (CH3OH) to investigate magnetic fields is a decade-old concept. The glow of the dust clouds that looks like natural microwave lasers, or masers is home to many newborn stars. The methanol masers signals are very strong and are of specific frequencies, which makes them easier to detect. The team had developed the model of methanol’s behaviour in such magnetic fields. And these data are collected from various telescopes.